Eye drop recall
Web 1 day agoHealth Manufacturer recalls eyedrops after possible link to bacterial infections Per the CDCs latest update 68 patients across 16 states have been infected with. Heres a running list.
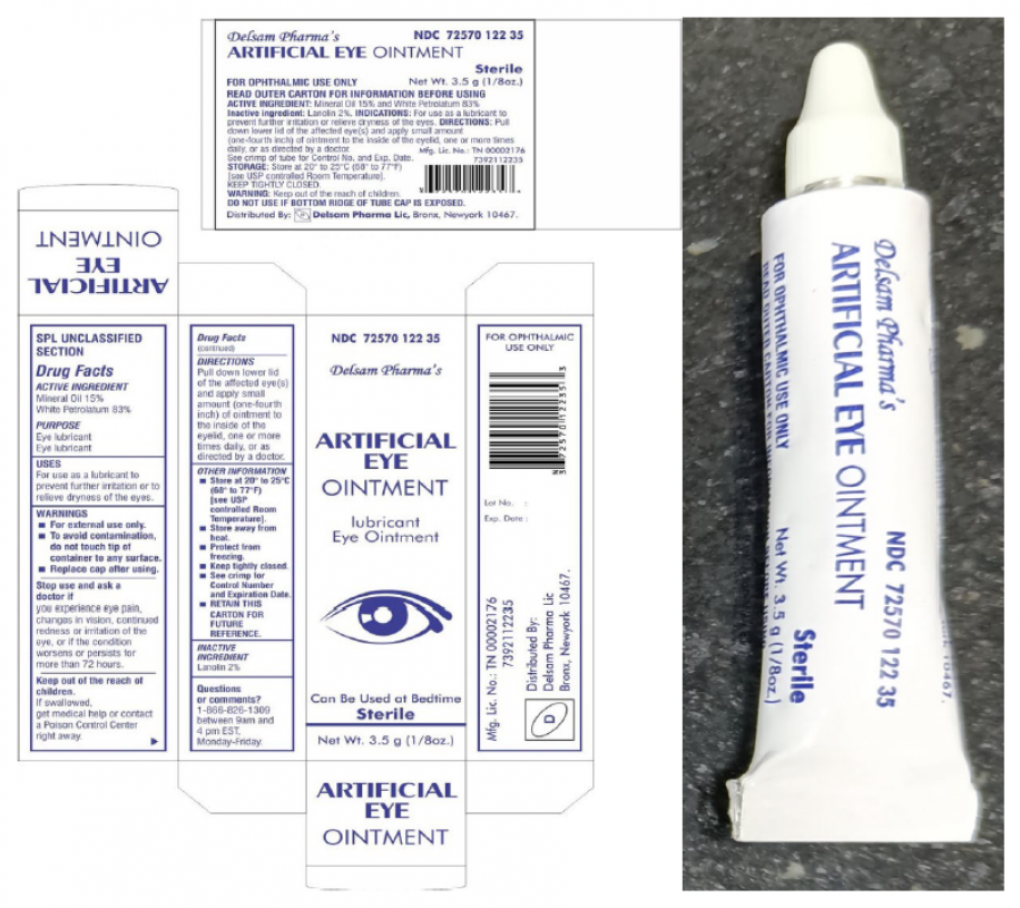
Delsam Pharma Eye Ointment Recalled Over Eye Infection Blindness Risk
- MarketWatch Multiple brands have recalled eye drops this year over concerns about.

. Web Most cases linked to the outbreak involved eye drops purchased online before the recall. Web The Food and Drug Administration posted separate recall notices for certain eyedrops distributed by Pharmedica and Apotex after the companies said they are. Web 5 hours agoTeva Pharmaceuticals has recalled nearly 716000 bottles of Clear Eyes eye drops for failed impurity testing according to The Miami Herald.
Web WASHINGTON US. Web Pharmedica USA issued a voluntary worldwide recall of two lots of its Purely Soothing 15 MSM eye drops the Food and Drug Administration announced March 3. Web At the time of the recall there were 55 reports of adverse reactions to the drops including eye infections permanent vision loss and one death from a.
Which cited an FDA. The manufacturer Global Pharma. Web A rare strain of bacteria found in recalled eyedrops has been linked to dozens of infections as well as cases of vision loss surgical removal of eyeballs and one.
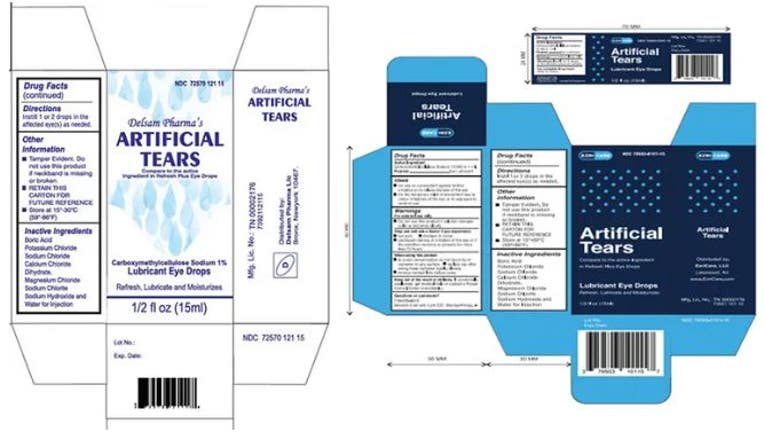
Web The FDA and CDC are advising patients to stop using EzriCare Artificial Tears an over-the-counter brand of eye drops. Pharmedica is recalling its Purely Soothing 15 MSM Drops. Web Review the recall notices.
Web The eye drops could be contaminated according to the recall and may be associated with the CDCs ongoing investigation into extensively drug-resistant. Web March 21 2023 349 PM. Five lots of Clear Eyes eye drops have been recalled by Teva Pharmaceuticals the latest in a run of recalls of prescription and over.
Web 11 hours agoPharmedica USA in March recalled Purely Soothing 15 MSM Drops out of concern that the product is not sterile. Said that as of March 14 the drug-resistant bacteria strain linked to the recalled EzriCare and Delsam eye drops had been. The CDC first alerted the public.
CNN Global Pharma Healthcare is issuing a recall. Web An alarming outbreak of extensively drug-resistant bacteria linked to eye drops has now sickened 68 people across 16 states according to the latest update from. Web The companies involved in the recalls are Phoenix-based Pharmedica and Florida-based Apotex.
Web Of those who were able to recall brand names 85 said theyd used preservative-free EzriCare Artificial Tears Walters said. Delsam Pharma eye drops are one of the brands subject to recall. Web According to the FDA the recalled eye drops were packaged in bottles with safety seals and small cartons with Ezricare drops having the NDC number 79503-0101.
Web 3 hours agoWhich eye drops have been recalled in 2023. Health officials are alerting consumers about two more recalls of eyedrops due to contamination risks that could lead to vision problems and. Web Global Pharma Healthcare is voluntarily recalling all lots within expiry of their Artificial Tears Lubricant Eye Drops distributed by EzriCare LLC- and Delsam Pharma to the.
However one reported buying EzriCare at a Costco warehouse. Web Updated 815 PM ET Thu February 2 2023. The drops have not been linked to illness the.
Why Are So Many Eye Drops Being Recalled Right Now Self
Pgn5ycba5ywmm

Eye Drop Recall Everything You Need To Know Right Now

Several Eye Drops Ointment Sold At Walgreens Walmart Recalled Ktve Myarklamiss Com
Clear Eyes Eye Allergy Itch Relief Eye Drops Recalled Miami Herald
Ucss80ity5 O2m

Eye Drops Are Recalled After Being Linked To Vision Loss And 1 Death The New York Times

Sw 3nggbwe6lvm
+1101902+eye+drops-Recall.jpg)
Fda Eye Drop Recall The Eye Associates
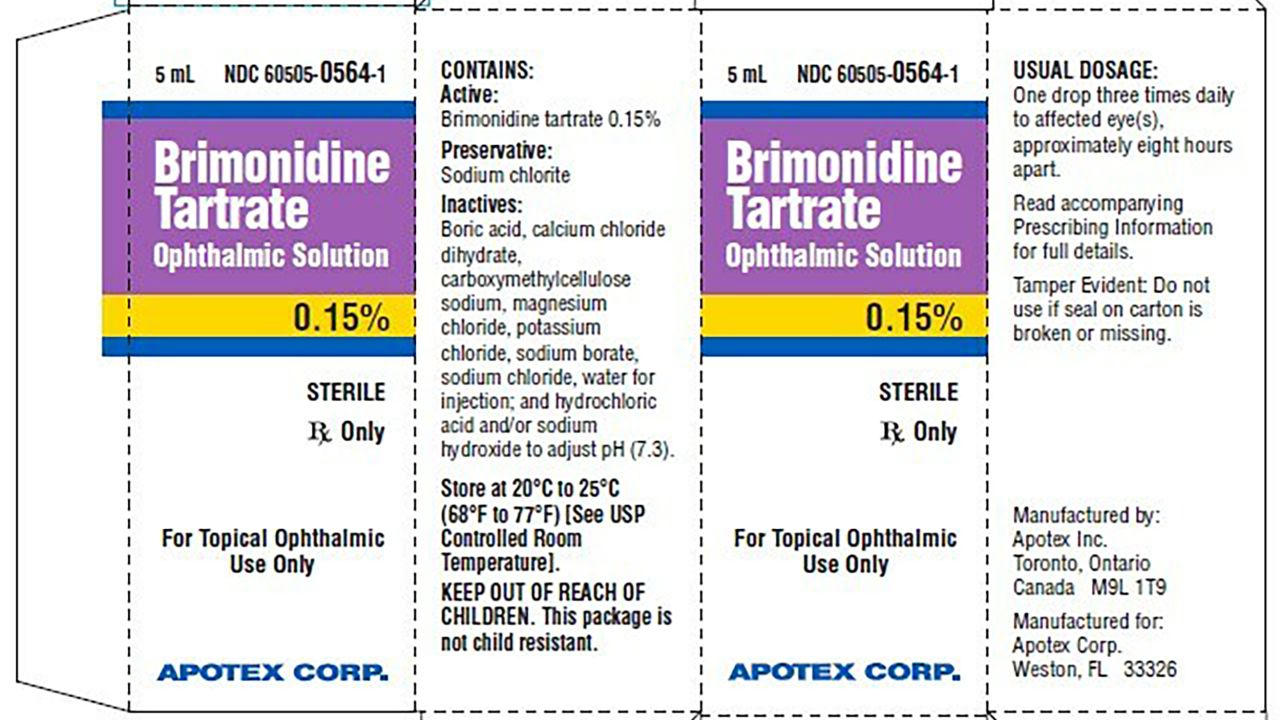
O N2vpc3liz1vm

Eye Infections From Tainted Eyedrops May Be More Widespread Doctors Worry
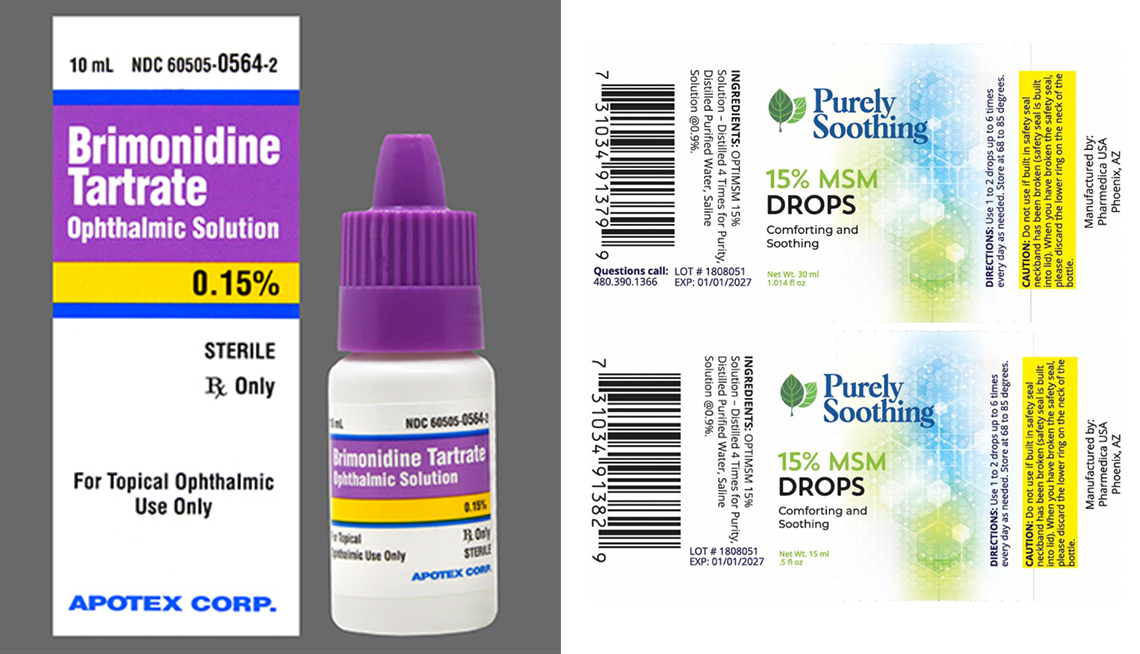
2 More Eye Drop Brands Recalled Due To Safety Risks
2 More Eye Drop Brands Recalled Due To Safety Risks

Lehmann Eye Center Check Your Medicine Cabinet Many Brands Of Eye Drops Prescription And Over The Counter Are Being Recalled For Potential Nonsterility At Present No Issues Have Been Reported However Use Of

Aezpbzfix Pwom

Chennai Firm Recalls Eye Drops After Us Flags Vision Loss Death

Eye Drops Recall Sparks Alarm As Doctors See Infections Linked To A Dangerous Bacteria